Blog post updated 6/19/23
Original publish date 2/21/18
How do you prevent adverse drug events (ADEs)? Even with protocols and automation, there are numerous challenges that confront medical professionals each day. In particular, dealing with allergies, juggling multiple medications and the tangle of tubing that exists when administering multiple IV infusions to a single patient, are all problematic.
And, patient safety data reinforces the ADE challenge. In fact, roughly one in 20 hospital patient's1 have experienced an adverse drug event 2. As a result, an essential element to patient safety and effective patient care is knowing how to properly label IV line tubing.
How To Properly Label IV Line Tubing
In fact, there is a higher risk and severity of error administering intraveneous (IV) medications than other medication adminstrations³. Experts believed that advances in technology—such as the use of so-called smart pumps—were the key to solving that problem. But even with technological advancements, errors still take place during the administration process.
Moreover, nearly 40% of medical errors take place at this stage 4 !
Although not all ADEs are preventable, it is generally estimated that almost half are 3 . So even with additional technology assisting the process, nearly 20% of medical errors could be prevented through more effective administration. And labeling IV line tubing properly is one of the keys to more effective administration.
Safe Medication Practices
As you would expect, the key to administration is to supply the correct medication to the correct patient at the correct time. In the hospital, this is generally a nurse or medical care provider's responsibility.
And when effective medication strategies are enacted, you can prevent adverse results.
Traditional safe medication practices have been taught using the five rights (administering the Right Medication, in the Right Dose, at the Right Time, by the Right Route, to the Right Patient).
But, because they contain no procedural detail, the five rights inadvertently promote the traditional focus on individual performance rather than system improvement 5 .
Procedures for ensuring each of the Five Rights must take into account the human factor and systems design issues. These include ineffective double check protocols, problems with patient wristbands and more that can threaten or undermine even the most conscientious efforts to comply with the five rights5 .
Multiple IV Lines & Infusions Increase Complexity
Systems design is especially important when patient complexity increases. Managing one IV and line is straightforward. But, when multiple IV bags and lines are involved, the complexity increases. Consequently, the question of how to label IV line tubing becomes even more important.
According to a report in Pharmacy Practice News that analyzed errors associated with multiple IV lines:
- Infusion rate or line mix-ups, IV lines not attaching to patients and errors associated with piggyback infusions were the most common errors associated with multiple IV infusions
- High-alert medications were involved in 71% of all multiple IV infusion errors and 92% of all IV line mix-ups
- Using the National Coordinating Council for Medication Error Reporting and Prevention, 48% of incidents were categorized as harm score D or greater, and 6.2% were categorized as harm score E or greater
- Nearly all (95%) of the errors reached the patient
Without ongoing vigilance, hospitals risk a sentinel event.
However, when the systems design and internal protocols include the use of IV Line & identification labels and training on how to correctly label IV line tubing, a reduction in unintended errors can occur. In fact, IV Line & identification labels assist the medical staff and guide the right dose at the right time by the right route.
Line & IV Labels
Furthermore, line and IV labels help manage the flow of medication into the IV lines. This ensures:
- Dispensing The Same Drug - IV bags and lines get tangled. A label placed at the top of the line and/or closest to the insertion point helps ensure proper dispensing
- Same Dose - Medication dosage is often changed from shift-to-shift. Noting the dose on line and bag safeguards consistent medication management
- Time managed - To ensure the patient receives proper medication dosage, start and end times are critical
In addition, labeling the IV bag with the appropriate medication label makes connecting the right IV line to that bag much simpler. In addition, consistent color coding patterns helps the staff to verify that lines are connected properly.
How To Properly Label IV Line Tubing
Depending upon where you are in the medication dispensing process will determine how to correctly label IV line tubing. For example,
Change Reminder Labels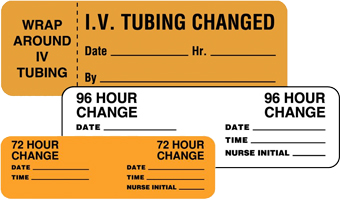
Standard protocols require regular IVs changes to prevent infection. A Change Reminder label alerts the medical staff that the IV to change it on a specific day or date. Also, when you use the information on a change reminder in conjunction with the drug type and medical chart, it helps guide the staff on appropriate patient actions. For example, when medication is added to the IV, noting the medication in several places ensures the communication is seen by all caregivers.
Line And IV Labels Enhance Patient Safety
Line and IV labels assist medication administration and simplify various medication management processes. These labels directly impact the medication administration errors that are common across healthcare settings. As a result, you can reduce the potential for mistakes.
Line & IV Labels Are Important For Joint Commission (TJC) Compliance
Effective labeling is an important element of the 2023 Joint Commission National Patient Safety Goals. In addition, The Joint Commission evaluates hospitals for consistency, medication safety and sentinel events. A lack of consistency can lead to serious medical consequences. However, Line & IV labels can help healthcare organizations maintain consistency, improve patient safety and meet Joint Commission standards.
Safe Handling of Hazardous Drugs (HDs)
IVs also present risks when used to administer hazardous drugs. To address these risks and other challenges associated with handling HDs, USP developed guidelines in their USP <800> chapter. The chapter recommends implementing policies to ensure safe handling such as:
- Separating IV HDs from other areas
- Clearly labeling the items with special handling instructions
- Using an externally ventilated, negative-pressure room with at least 12 air changes per hour (ACPH)
In addition, if transportation occurs outside the facility, Safety Data Sheet (SDS) and labels detailing HD storage and disposal instructions are required. Developed to protect the environment, health care workers, and patients from exposure to hazardous agents, USP <800> guidelines become official on November 1, 2023.
UAL supplies labels that help ensure both worker and patient safety and conform to USP <800> guidelines.
United Ad Label Has Extensive IV Line Label Expertise
United Ad Label has extensive experience with IV Line labeling. We regularly work with healthcare organizations to understand how to label IV line tubing properly. This will help ensure both Joint Commission standards and internal protocols are met. You can read about our work with one hospital system here.
Editor's Note: This post was updated in June, 2023 to reflect the latest best practices on IV line tubing and medication safety.