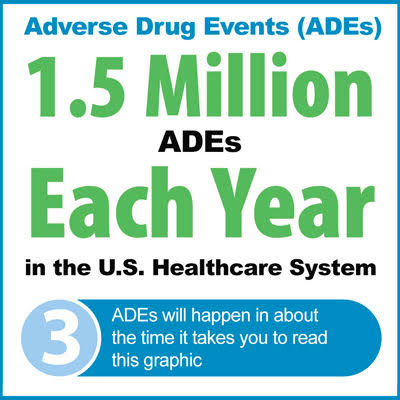
Do IV Line Labels contribute to adverse drug events (ADEs)?
As detailed in the The Importance of IV & Line Labels For Effective Care, roughly one in 20 hospital patient’s1 have experienced an adverse drug event.2 And, one of the most common types of hospital errors occur during the administration of intravenous (IV) medications.2
IV Line Labeling Challenges
IV line labeling challenges can contribute to medical errors. Although it doesn’t seem complex, there are numerous interrelated processes connected with IV line labeling including: Medication Ordering Preparation Distribution Administration This interrelationship creates challenges for medical staff because, stakeholders at each stage of the process have different information needs. Unfortunately, in an attempt to fulfill those diverse needs, the labels often contain an overabundance of data. This article details a few of the common challenges and what to look out for to ensure there’s no unintended patient safety lapse.
IV Line Labels
Although they have a small footprint, IV Line labels communicate substantial information to medical professionals during patient care. They help:
- Monitor IV fluid levels Prevent medication line mix ups
- Organize multiple drips
- Highlight medications added to the IV Ensure IV site care
- Highlight when an IV change is due
- Convey medical alerts
Because these are common needs across all hospitals, healthcare label providers like United Ad Label, have worked with healthcare providers to develop labels that fit their requirements. Those applications include:
- IV line identification
- Medication added Flow strips
- Change minders
- Alerts and Observation
Click here to see these and other labels that are commonly used for these applications. The nursing staff is the most common user of IV line labels but other medical professionals including:
- Doctors
- Lab Technicians
- Pharmacists
- Clinicians
- And More
Use the information to guide patient care.
IV Line Labels Are Both Basic And Complex
An IV line label is commonly viewed as small and simplistic. In reality, they contain numerous complexities.
For example, IV bags require a label that’s sized to fit on the smallest bag. But, the need to communicate important information stays the same, even with the space limitations.
In addition, selecting the right materials and ink are important for label storage. Without the right adhesives, the label can shift or fall off in the refrigerator. In addition, labels that are designed for the cold temperatures of a refrigerator, without consideration for printing, become problematic when run through a laser printer.
But it’s not just hot and cold temperatures. Nurses administer medications in a variety of environments both dark and bright. A white label that works fine in limited lighting is difficult to read when placed on an IV that’s near a window with bright sunshine.
Although, healthcare label providers like United Ad Label, have worked with healthcare facilities to develop stock labels that fit their requirements, internal needs and protocols may necessitate developing a customized solution.
Stakeholder Requirements
Physicians, nurses and pharmacists use the same label but for different purposes. Information required by pharmacists ends up intermixed with information that nurses need for administering the order.
Plus, internal systems and technical challenges may also inhibit developing the perfect IV line label. For example, the pharmacy and lab system software dictate the format and positioning of the data placed on the label.
In addition, CPOE systems such as EPIC, Meditech, McKesson, VA and others have a set of data they customize for each hospital that is consistent with their protocol and medical needs. Some facilities have bedside medication verification and others do not. In the case of bedside verification an additional barcode is printed on the medication label.
Lastly, the orders a physician issues may complicate the process further. For example, it may not be possible to flag individual fields like the drug name or print them in a particular location or contrasting font. Unfortunately, this requires each stakeholder to search for the information that is relevant to his or her tasks.
Gaining stakeholder commitment requires a plan that consolidated the needs of risk management, nurses and patients. Identifying unique line and medication labels used throughout the facility and the various lines used on each label and the data input each one required are the first steps.
Contractual Arrangements
As providers look for savings across all areas of procurement, it’s common to buy a larger quantity of medical labels to reduce the overall unit cost. In addition, materials management may look at stock items as a savings opportunity. Although these tactics reduce the overall spend, depending upon your internal requirements, they may not be the best option. A poor functioning label results in a less efficient nursing staff and a higher risk of medical error.
However, if internal protocols and compliance needs are met, then the less expensive stock items may be a good fit.
IV Line Label Compliance Requirements
Both nurses and pharmacists use the same label but for different purposes. Unfortunately, the information required by pharmacists ends up intermixed with information that nurses need for administering the order. Each stakeholder must search for the information that is relevant to his or her tasks. It also leads to blank lines.
This may create a compliance issue. When a line isn’t relevant to a specific stakeholder leaving it blank is a common outcome. But, the Joint Commission requires information on each line.
Steps For IV Line Label Improvement
Even with the complexities of processes, stakeholders and systems, there are steps you can take to improve the IV line label system. Our next post will detail improvement process steps available to all providers. Subscribe to our newsletter to get the next installment delivered to your inbox. This is the second in a four part series that details how you can use IV Line labels more effectively to improve patient outcomes. Visit our blog to read the first post if you missed it.
Click here to review UAL IV line labels.
Footnotes
2An ADE refers to any injury occurring at the time a drug is used, whether or not it is identified as a cause of the injury.
2http://www.aami.org/newsviews/newsdetail.aspx?ItemNumber=3149