Since last March, COVID-19 has created a series of ongoing challenges for healthcare providers. Fortunately, FDA approval of the Pfizer and Moderna COVID-19 vaccines and the expectation that over 100 million people in the U.S. will receive it by March 31, 2021, offers hope that the end of the pandemic is in sight. But, administering the vaccine to millions of people won’t be easy. In fact, storing and handling the vaccine properly will create yet one more hurdle for healthcare providers to overcome. Use these COVID-19 vaccine storage tips to help you manage the process.
COVID-19 Vaccine Storage Tips
 The COVID-19 vaccine storage process is different from other vaccines because of the cold storage requirements. The Pfizer version requires -70℃ temperature storage until it’s removed from the shipping containers. If ultra-low temperature freezers aren’t available, replenishing the dry ice in the shipping container every five days will maintain the temperature properly for up to 30 days.
The COVID-19 vaccine storage process is different from other vaccines because of the cold storage requirements. The Pfizer version requires -70℃ temperature storage until it’s removed from the shipping containers. If ultra-low temperature freezers aren’t available, replenishing the dry ice in the shipping container every five days will maintain the temperature properly for up to 30 days.
The Moderna vaccine doesn’t require the same extreme cold for storage, only -25°C and -15°C. But, this is a tighter temperature range than other vaccines and requires monitoring to ensure those thresholds are maintained.
Cold Storage Safety
If you don’t have adequate cold storage and plan on using dry ice, you need to take specific precautions including:
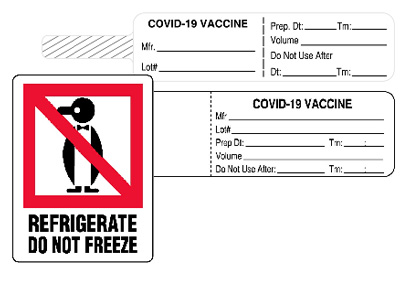
- Using temperature indicating labels that allow you to ensure proper temperature thresholds are met
- Destroying doses that violate these temperature thresholds. In fact, maintaining proper temperatures caused problems for both the Pfizer and Moderna vaccines.
- Wearing proper PPE including gloves and goggles when handling dry ice
- Use contact hazard labels to alert staff about using proper precautions
In addition, once opened, both the Pfizer and Moderna vaccines require similar refrigeration temperatures at 2 to 8℃. You can refrigerate the Pfizer version for up to five days and Moderna for 30. Plus, after reconstituting the Pfizer vaccine, it must be used within six hours.
To ensure you uphold these requirements:
- Mark cartons and containers with keep refrigerated labels
- Mark vials and syringes with COVID-19 syringe and flag labels
COVID-19 Vaccine Handling
In addition to the precautions necessary around expiration hours and dates, extra care is also required when reconstituting vials. Each vaccine requires tumbling, not shaking, Shaking disrupts the chemistry.
Using communication labels promotes proper handling and informs the medical staff of appropriate protocols. Plus, handing out “I Got The COVID-19 Vaccine” stickers will encourage others to get their vaccine.
United Ad Label
These COVID-19 vaccine storage tips will help you manage the vaccination workflows. United Ad Label supplies stock and custom labels that allow healthcare organizations to safely store and handle COVID-19 vaccines. Contact us to learn more.